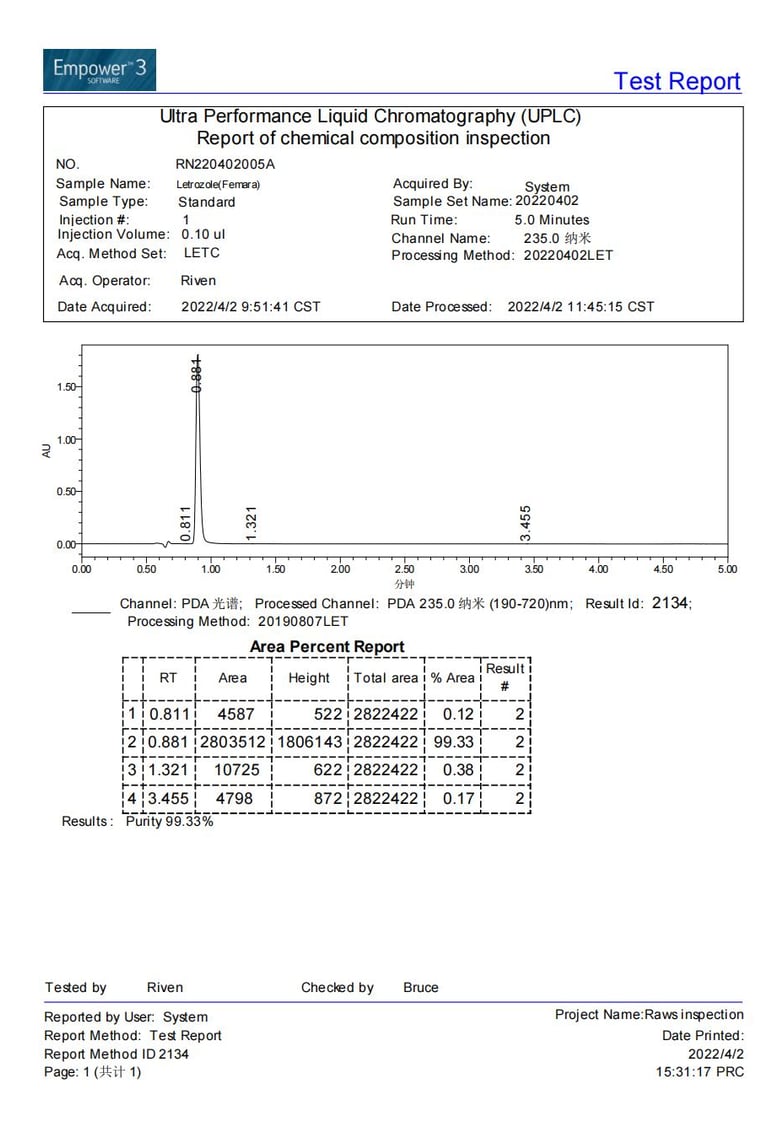
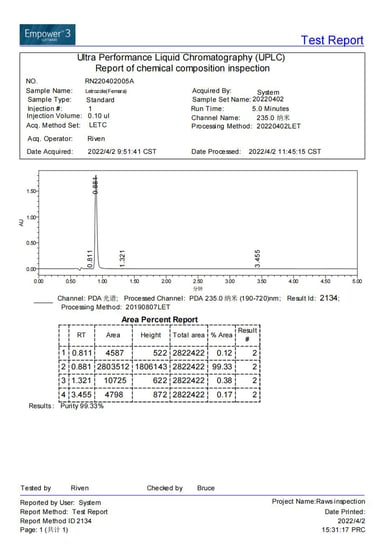
Current stock purity 99.33% tested on 2022/04/02
Currently produced in 2.5mg Capsules. Quantity per container will vary per distributor.
Letrozole, sold under the brand name Femara among others, is an aromatase inhibitor which is used in the treatment of hormonally-responsive breast cancer after surgery.
It was patented in 1986 and approved for medical use in 1996.
Letrozole is approved by the United States Food and Drug Administration (FDA) for the treatment of local or metastatic breast cancer that is hormone receptor positive or has an unknown receptor status in postmenopausal women.
Comparison with tamoxifen
Tamoxifen is also used to treat hormonally-responsive breast cancer, but it does so by interfering with the estrogen receptor. However, letrozole is effective only in post-menopausal women, in whom estrogen is produced predominantly in peripheral tissues (i.e. in adipose tissue, like that of the breast) and a number of sites in the brain. In pre-menopausal women, the main source of estrogen is from the ovaries not the peripheral tissues, and letrozole is ineffective.
In the BIG 1–98 Study, of post-menopausal women with hormonally-responsive breast cancer, letrozole reduced the recurrence of cancer, but did not change survival rate, compared to tamoxifen.
The most common side effects are sweating, hot flashes, arthralgia (joint pain), and fatigue.
Generally, side effects include signs and symptoms of hypoestrogenism. There is concern that long term use may lead to osteoporosis, which is in certain patient populations such as post-menopausal women or osteoporotics, bisphosphonates may also be prescribed.